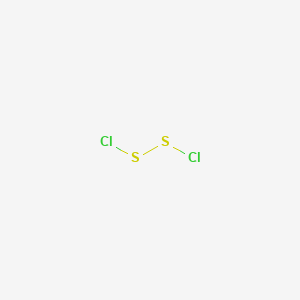
Disulfur dichloride s 2 cl 2 is the most common impurity in scl 2.
Sulfur dichloride state at room temperature.
Sulfur is a solid at room temperature.
Pure disulfur dichloride is a yellow liquid that smokes in moist air due to reaction with water.
Is state of matter a physical or a chemical.
High pressure solid allotropes.
In a high pressure study at ambient temperatures four new solid forms termed ii iii iv v have been characterized where α sulfur is form i.
Sulfuryl chloride is commonly confused with thionyl chloride socl 2 the properties of these two sulfur oxychlorides are quite different.
Sulfuryl chloride is a source of.
Sulfur dichloride worksites should be equipped with emergency showers and eyewash equipment for this purpose.
No it s not a change in the state of matter is a physical change if that s what you were trying to ask.
Sulfur dioxide is a colorless gas with a pungent odor.
An idealized but complicated equation is.
16 s 2 cl 2 16 h 2 o 8 so 2 32 hcl 3 s 8.
Contaminated clothing and shoes should be removed and left at the worksite.
In nature sulfur dioxide can be released to the air from volcanic eruptions.
The region labeled i a solid region is α sulfur.
Sulfuryl chloride is an inorganic compound with the formula so 2 cl 2 at room temperature it is a colorless liquid with a pungent odor sulfuryl chloride is not found in nature as can be inferred from its rapid hydrolysis.
The reaction proceeds at usable rates at room temperature.
While sulfur dichloride does not burn easily it may ignite other combustible materials eg wood paper oil.
Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
It is a liquid when under pressure and it dissolves in water very easily.
Solid forms ii and iii are polymeric while iv and v are metallic and are.
Relative atomic mass the mass of an atom relative to that of.
It is produced by partial chlorination of elemental sulfur.
Separation of scl 2 from s 2 cl 2 is possible via distillation with pcl 3 to form an azeotrope of 99 purity however sulfur dichloride loses chlorine slowly at room temperature.
The pressure temperature p t phase diagram for sulfur is complex see image.
The temperature at which the liquid gas phase change occurs.